Geographic Atrophy Market to Surge Significantly During the Forecast Period (2025–2034) Due to Rising Adoption of Complement Inhibitor Therapies | DelveInsight
The dynamics of the geographic atrophy market are expected to change in the coming years due to the rising global prevalence driven by aging populations, demand for disease-modifying interventions, and the launch of emerging therapies such as Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), AVD-104 (Aviceda Therapeutics), JNJ-1887 (Johnson & Johnson Innovative Medicine), OGX-110 (Ocugenix), OCU410 (Ocugen), BI 1584862 (Boehringer Ingelheim), and others.
New York, USA, Dec. 01, 2025 (GLOBE NEWSWIRE) -- Geographic Atrophy Market to Surge Significantly During the Forecast Period (2025–2034) Due to Rising Adoption of Complement Inhibitor Therapies | DelveInsight
The dynamics of the geographic atrophy market are expected to change in the coming years due to the rising global prevalence driven by aging populations, demand for disease-modifying interventions, and the launch of emerging therapies such as Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), AVD-104 (Aviceda Therapeutics), JNJ-1887 (Johnson & Johnson Innovative Medicine), OGX-110 (Ocugenix), OCU410 (Ocugen), BI 1584862 (Boehringer Ingelheim), and others.
DelveInsight’s Geographic Atrophy Market Insights report includes a comprehensive understanding of current treatment practices, emerging geographic atrophy drugs, market share of individual therapies, and current and forecasted geographic atrophy market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
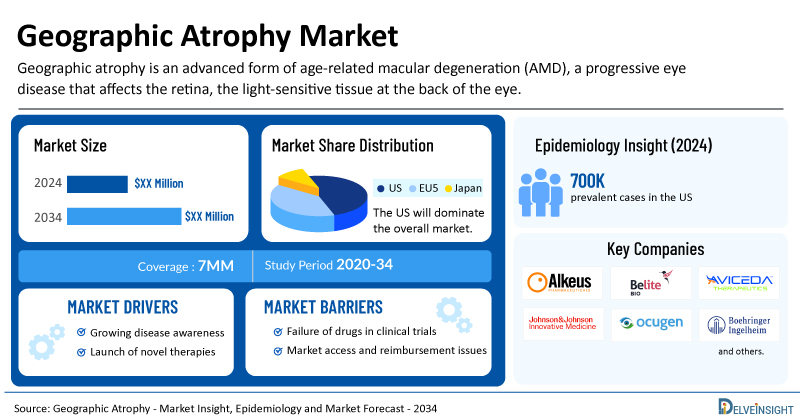
Geographic Atrophy Market Summary
- The total geographic atrophy treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of geographic atrophy, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- The US accounted for around 700K prevalent cases of geographic atrophy in 2024.
- Key geographic atrophy companies, including Alkeus Pharmaceuticals, Belite Bio, Aviceda Therapeutics, Johnson & Johnson Innovative Medicine, Ocugenix, Ocugen, Boehringer Ingelheim, Lineage Cell Therapeutics (CellCure Neurosciences), Roche, Cognition Therapeutics, Stealth BioTherapeutics, Evergreen Therapeutics, Annexon Biosciences, NGM Biopharmaceuticals, AstraZeneca, Alexion Pharmaceuticals, Ionis Pharmaceuticals, Regenerative Patch Technologies, and others, are actively working on innovative geographic atrophy drugs.
- Some of the key geographic atrophy therapies in clinical trials include Gildeuretinol, Tinlarebant, AVD-104, JNJ-1887, OGX-110, OCU410, BI 1584862, OpRegen, CT1812, Elamipretide, EG-301, ANX007, NGM621, Danicopan (ALXN2040), IONIS-FB-LRx, CPCB-RPE1, and others. These novel Geographic Atrophy therapies are anticipated to enter the geographic atrophy market in the forecast period and are expected to change the market.
Discover which geographic atrophy medications are expected to grab the market share @ Geographic Atrophy Market Report
Key Factors Driving the Growth of the Geographic Atrophy Market
Rising Geographic Atrophy Prevalence
The prevalence of geographic atrophy, an advanced form of age-related macular degeneration, is steadily rising due to global population ageing and increased life expectancy. As more individuals enter older age groups, the number of those at risk for geographic atrophy has grown significantly.
Emerging Diagnostic Technologies in Geographic Atrophy
New diagnostic technologies, such as spectral-domain OCT and fundus autofluorescence, have emerged, giving ophthalmologists a clearer picture of the eye and aiding the confirmation of anatomic endpoints for geographic atrophy.
ANX007 Milestone in Geographic Atrophy
ANX007 is the first therapeutic candidate for the treatment of geographic atrophy to receive PRIME designation in the EU, which provides early and proactive support to developers of promising medicines that may offer a major therapeutic advantage over existing treatments or benefit patients without treatment options.
Evidence Supporting RPE Cell Therapy for Geographic Atrophy
Data from both CPCB-RPE1 and OpRegen provide a very strong likelihood that RPE cells are an effective means of treating geographic atrophy.
Launch of Emerging Geographic Atrophy Drugs
The launch of geographic atrophy drugs such as Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), AVD-104 (Aviceda Therapeutics), JNJ-1887 (Johnson & Johnson Innovative Medicine), OGX-110 (Ocugenix), OCU410 (Ocugen), BI 1584862 (Boehringer Ingelheim), JNJ-1887 sCD59 (Janssen Research & Development, LLC), OpRegen (Lineage Cell Therapeutics (CellCure Neurosciences) and Roche), CT1812 (Cognition Therapeutics), Elamipretide (Stealth BioTherapeutics), EG-301 (Evergreen Therapeutics), ANX007 (Annexon Biosciences), NGM621 (NGM Biopharmaceuticals), Danicopan (ALXN2040) (AstraZeneca/Alexion Pharmaceuticals), IONIS-FB-LRx (Ionis Pharmaceuticals/Roche), CPCB-RPE1 (Regenerative Patch Technologies), and others are expected to change the dynamics of the geographic atrophy market in the coming years.
Geographic Atrophy Market Analysis
Agents that modulate the immune response have become the most advanced segment within clinical research for geographic atrophy, marking a major milestone as the first and only class of therapies to reach the market so far. The FDA approvals of IZERVAY (avacincaptad pegol) and SYFOVRE (pegcetacoplan) represent significant breakthroughs in this space.
While IZERVAY and SYFOVRE offer new hope for individuals with late-stage AMD, they also represent only the beginning of therapeutic progress for geographic atrophy. Safety-related setbacks temporarily slowed SYFOVRE’s early momentum and dampened its initial market lead. Nevertheless, Apellis reported strong Q4 sales, demonstrating robust growth for SYFOVRE. Looking ahead, with several new therapies expected to seek regulatory approval between 2025 and 2027, the evolution of the geographic atrophy treatment landscape will be worth watching. For now, SYFOVRE and IZERVAY face no immediate competitive threats, as no other products are nearing approval.
Surgical approaches for geographic atrophy management include the PRIMA Bionic Vision System, the SING-IMT implant, and the OcuDyne neuro-interventional procedure. A wide range of therapies is currently in development to slow disease progression and preserve macular function in dry AMD. Among the promising candidates under investigation are ALK-001, Tinlarebant, Elamipretide, and OGX-110. Many of these aim to prevent disease onset or halt worsening.
Given the older demographic affected by geographic atrophy, treatments with every-other-month dosing may see higher demand. As a result, options offering monthly dosing, or even a one-time administration, could capture meaningful market share. With a large patient population and considerable unmet need, future therapies have significant potential to become the standard of care.
Learn more about the geographic atrophy treatment options @ Geographic Atrophy Treatment Market
Geographic Atrophy Competitive Landscape
Some of the geographic atrophy drugs in clinical trials include Gildeuretinol (Alkeus Pharmaceuticals), Tinlarebant (Belite Bio), AVD-104 (Aviceda Therapeutics), JNJ-1887 (Johnson & Johnson Innovative Medicine), OGX-110 (Ocugenix), OCU410 (Ocugen), BI 1584862 (Boehringer Ingelheim), JNJ-1887 sCD59 (Janssen Research & Development, LLC), OpRegen (Lineage Cell Therapeutics (CellCure Neurosciences) and Roche), CT1812 (Cognition Therapeutics), Elamipretide (Stealth BioTherapeutics), EG-301 (Evergreen Therapeutics), ANX007 (Annexon Biosciences), NGM621 (NGM Biopharmaceuticals), Danicopan (ALXN2040) (AstraZeneca/Alexion Pharmaceuticals), IONIS-FB-LRx (Ionis Pharmaceuticals/Roche), CPCB-RPE1 (Regenerative Patch Technologies), and others.
Alkeus Pharmaceuticals’ Gildeuretinol (ALK-001) is an oral, once-daily investigational therapy for geographic atrophy. It is a chemically modified form of vitamin A designed to limit the buildup of toxic by-products that contribute to retinal degeneration. In addition to geographic atrophy, ALK-001 is also being tested for potential use in Stargardt disease.
Belite Bio’s Tinlarebant is an innovative oral drug designed to reduce harmful by-products of the visual cycle that drive STGD1 and contribute to geographic atrophy, an advanced form of dry AMD. A global, two-year Phase III trial (PHOENIX) is currently underway. This randomized, double-masked, placebo-controlled study is assessing Tinlarebant’s safety and effectiveness in patients with geographic atrophy. In November 2023, Belite Bio received MHRA approval in the UK to proceed with this Phase III study. The company plans an interim analysis at the midpoint of the trial.
Ocugenix’s OGX-110, formerly known as Ocu-110, is a small-molecule therapy that precisely targets the CXCR3 signaling pathway—an essential regulator of tissue repair that helps remove abnormal blood vessels, limit fibrosis, and restore the balance of wound healing. By binding to the natural CXCR3 receptor site, OGX-110 promotes a regenerative healing response, counteracting pro-angiogenic and pro-fibrotic factors such as VEGF. The therapy is currently in Phase I clinical testing.
The anticipated launch of these emerging geographic atrophy therapies are poised to transform the geographic atrophy market landscape in the coming years. As these cutting-edge geographic atrophy therapies continue to mature and gain regulatory approval, they are expected to reshape the geographic atrophy market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for geographic atrophy, visit @ Geographic Atrophy Medication
Recent Developments in the Geographic Atrophy Market
- In November 2025, Apellis Pharmaceuticals, Inc. released findings from a post hoc analysis of the GALE extension study, showing results after five years of ongoing treatment with SYFOVRE (pegcetacoplan injection), the leading therapy for geographic atrophy caused by age-related macular degeneration (AMD).
- In October 2025, ONL Therapeutics, Inc. reported that it had enrolled and randomized the first participant in its Phase 2 GALAXY study. This global trial is assessing the safety and effectiveness of xelafaslatide (previously known as ONL1204) for treating patients with geographic atrophy linked to dry age-related macular degeneration (AMD).
- In October 2025, Astellas Pharma Inc. reported initial findings from the open-label extension of the Phase 3 GATHER2 trial, showing that monthly dosing of IZERVAY (avacincaptad pegol intravitreal solution) in patients with geographic atrophy due to age-related macular degeneration (AMD) sustained reductions in GA lesion growth for as long as 3.5 years, with earlier treatment offering stronger preservation of retinal tissue.
- In October 2025, Complement Therapeutics GmbH reported that the FDA had approved its Investigational New Drug (IND) application for CTx001, the company’s flagship gene therapy candidate.
- In July 2025, Belite bio announces completion of enrollment in the pivotal global phase III PHOENIX trial evaluating oral tinlarebant in geographic atrophy.
What is Geographic Atrophy?
Geographic atrophy is an advanced form of age-related macular degeneration (AMD), a progressive eye disease that affects the retina, the light-sensitive tissue at the back of the eye. In geographic atrophy, areas of retinal cells, including the photoreceptors, retinal pigment epithelium (RPE), and underlying choriocapillaris, gradually degenerate, creating well-defined patches of vision loss. These areas expand over time, leading to difficulties with tasks such as reading, recognizing faces, and seeing in low light. Although geographic atrophy does not typically cause complete blindness, it can severely impair central vision and quality of life. Currently, geographic atrophy remains a chronic, irreversible condition, but emerging therapies aim to slow its progression.
Geographic Atrophy Epidemiology Segmentation
The geographic atrophy epidemiology section provides insights into the historical and current geographic atrophy patient pool and forecasted trends for the leading markets. In geographic atrophy cases, moderate-to-severe visual impairment is predominant, accounting for more than 40%, while occurrences requiring surgery or leading to legal blindness are less common.
The geographic atrophy market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Prevalent Cases of AMD
- Late Stage-specific Prevalent Cases of AMD
- Total Prevalent Cases of Geographic Atrophy
- Total Diagnosed Prevalent Cases of Geographic Atrophy
- Age-specific Cases of Geographic Atrophy
- Geographic Atrophy Cases by Visual Impairment
- Total Treated Cases of Geographic Atrophy
Download the report to understand geographic atrophy management @ Geographic Atrophy Treatment Options
| Geographic Atrophy Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Geographic Atrophy Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Geographic Atrophy Epidemiology Segmentation | Total Prevalent Cases of AMD, Late Stage-specific Prevalent Cases of AMD, Total Prevalent Cases of Geographic Atrophy, Total Diagnosed Prevalent Cases of Geographic Atrophy, Age-specific Cases of Geographic Atrophy, Geographic Atrophy Cases by Visual Impairment, and Total Treated Cases of Geographic Atrophy |
| Key Geographic Atrophy Companies | Alkeus Pharmaceuticals, Belite Bio, Aviceda Therapeutics, Johnson & Johnson Innovative Medicine, Ocugenix, Ocugen, Boehringer Ingelheim, Lineage Cell Therapeutics (CellCure Neurosciences), Roche, Cognition Therapeutics, Stealth BioTherapeutics, Evergreen Therapeutics, Annexon Biosciences, NGM Biopharmaceuticals, AstraZeneca, Alexion Pharmaceuticals, Ionis Pharmaceuticals, Regenerative Patch Technologies, Astellas Pharma, Iveric Bio, Apellis Pharmaceuticals, and others |
| Key Geographic Atrophy Therapies | Gildeuretinol, Tinlarebant, AVD-104, JNJ-1887, OGX-110, OCU410, BI 1584862, OpRegen, CT1812, Elamipretide, EG-301, ANX007, NGM621, Danicopan (ALXN2040), IONIS-FB-LRx, CPCB-RPE1, IZERVAY, SYFOVRE, and others |
Scope of the Geographic Atrophy Market Report
- Geographic Atrophy Therapeutic Assessment: Geographic Atrophy current marketed and emerging therapies
- Geographic Atrophy Market Dynamics: Conjoint Analysis of Emerging Geographic Atrophy Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Geographic Atrophy Market Unmet Needs, KOL’s views, Analyst’s views, Geographic Atrophy Market Access and Reimbursement
Discover more about geographic atrophy drugs in development @ Geographic Atrophy Clinical Trials
Table of Contents
| 1 | Geographic Atrophy Market Key Insights |
| 2 | Geographic Atrophy Market Report Introduction |
| 3 | Executive Summary of Geographic Atrophy |
| 4 | Key Events |
| 5 | Epidemiology and Market Forecast Methodology |
| 6 | Geographic Atrophy Market Overview at a Glance |
| 6.1 | Market Share by Therapies (%) Distribution of Geographic Atrophy in 2024 in the 7MM |
| 6.2 | Market Share by Therapies (%) Distribution of Geographic Atrophy in 2034 in the 7MM |
| 7 | Disease Background and Overview |
| 7.1 | Introduction |
| 7.2 | Geographic Atrophy Diagnosis |
| 7.3 | Geographic Atrophy Diagnostic Guidelines |
| 7.4 | vTreatment |
| 7.5 | Geographic Atrophy Treatment Guidelines |
| 8 | Epidemiology and Patient Population |
| 8.1 | Key Findings |
| 8.2 | Assumption and Rationale |
| 8.3 | Total Prevalent Cases of AMD in the 7MM |
| 8.4 | Total Prevalent Cases of Geographic Atrophy in the 7MM |
| 8.5 | The United States |
| 8.5.1 | Total Prevalent Cases of AMD in the United States |
| 8.5.2 | Late Stage-specific Prevalent Cases of AMD in the United States |
| 8.5.3 | Total Prevalent Cases of Geographic Atrophy in the United States |
| 8.5.4 | Total Diagnosed Prevalent Cases of Geographic Atrophy in the United States |
| 8.5.5 | Age-specific Cases of Geographic Atrophy in the United States |
| 8.5.6 | Geographic Atrophy Cases by Visual Impairment in the United States |
| 8.5.7 | Total Treated Cases of Geographic Atrophy in the United States |
| 8.6 | EU4 and the UK |
| 8.7 | Japan |
| 9 | Geographic Atrophy Patient Journey |
| 9.1 | Description |
| 10 | Marketed Geographic Atrophy Drugs |
| 10.1 | Key Competitor |
| 10.2 | IZERVAY (avacincaptad pegol): Astellas Pharma/Iveric Bio |
| 10.2.1 | Product Description |
| 10.2.2 | Regulatory Milestones |
| 10.2.3 | Other Developmental Activities |
| 10.2.4 | Summary of Pivotal Trials |
| 10.2.5 | Safety and Efficacy |
| 10.2.6 | Analyst Views |
| 10.3 | SYFOVRE (pegcetacoplan): Apellis Pharmaceuticals |
| 11 | Emerging Geographic Atrophy Drugs |
| 11.1 | Key Competitors |
| 11.2 | Gildeuretinol (ALK-001): Alkeus Pharmaceuticals |
| 11.2.1 | Product Description |
| 11.2.2 | Other Developmental Activities |
| 11.2.3 | Clinical Developmental Activities |
| 11.2.3.1 | Clinical Trial Information |
| 11.2.4 | Safety and Efficacy |
| 11.2.5 | Analyst Views |
| 11.3 | Tinlarebant (LBS-008): Belite Bio |
| 11.4 | AVD-104: Aviceda Therapeutics |
| 11.5 | OGX-110: Ocugenix |
| 11.6 | OpRegen: Lineage Cell Therapeutics (CellCure Neurosciences) and Roche |
| 11.7 | CT1812: Cognition Therapeutics |
| 11.8 | Elamipretide: Stealth BioTherapeutics |
| 11.9 | EG-301: Evergreen Therapeutics |
| 11.10 | ANX007: Annexon Biosciences |
| 11.11 | NGM621: NGM Biopharmaceuticals |
| 11.12 | Danicopan (ALXN2040): AstraZeneca/Alexion Pharmaceuticals |
| 11.13 | IONIS-FB-LRx: Ionis Pharmaceuticals/Roche |
| 11.14 | CPCB-RPE1: Regenerative Patch Technologies |
| 12 | Geographic Atrophy: Market Analysis |
| 12.1 | Key Findings |
| 12.2 | Geographic Atrophy Market Outlook |
| 12.3 | Conjoint Analysis |
| 12.4 | Key Geographic Atrophy Market Forecast Assumptions |
| 12.5 | Total Market Size of Geographic Atrophy in the 7MM |
| 12.6 | United States Geographic Atrophy Market Size |
| 12.6.1 | Total Market Size of Geographic Atrophy in the United States |
| 12.6.2 | Market Size of Geographic Atrophy by Therapies in the United States |
| 12.7 | EU4 and the UK Geographic Atrophy Market Size |
| 12.8 | Japan Geographic Atrophy Market Size |
| 13 | Geographic Atrophy Market Unmet Needs |
| 14 | Geographic Atrophy Market SWOT Analysis |
| 15 | KOL Views on Geographic Atrophy |
| 16 | Geographic Atrophy Market Access and Reimbursement |
| 16.1 | United States |
| 16.2 | EU4 and the UK |
| 16.3 | Japan |
| 16.4 | Market Access and Reimbursement of Geographic Atrophy |
| 17 | Bibliography |
| 18 | Geographic Atrophy Market Report Methodology |
Related Reports
Geographic Atrophy Clinical Trial Analysis
Geographic Atrophy Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key geographic atrophy companies, including Ionis Pharmaceuticals, IVERIC bio, Apellis Pharmaceuticals, Inc., NGM Biopharmaceuticals, Annexon Inc., Genentech, Alexion AstraZeneca Rare Disease, ONL Therapeutics, Alkeus Pharmaceuticals, Regenerative Patch Technologies, LLC, Astellas Pharma Inc., Gemini Therapeutics, Boehringer Ingelheim, Cell Cure Neurosciences LTD, Stealth BioTherapeutics Inc., Hemera Biosciences LLC, Gyroscope Therapeutics Limited, Eyevensys, Nanoscope Therapeutics, Inc., Catalyst Biosciences, Novartis, among others.
Age-related Macular Degeneration Market
Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key AMD companies, including Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Evergreen Therapeutics, Alkeus Pharmaceuticals, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Novartis, Luxa Biotechnology, Gemini Therapeutics, among others.
Age-related Macular Degeneration Clinical Trial Analysis
Age-related Macular Degeneration Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key age-related macular degeneration companies including Regeneron Pharmaceuticals, Novartis, Roche, Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Graybug Vision, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Evergreen Therapeutics, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Luxa Biotechnology, Gemini Therapeutics, among others.
Dry Age-related Macular Degeneration Market
Dry Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key dry AMD companies including Alkeus Pharmaceuticals, Belite Bio, Aviceda Therapeutics, Johnson & Johnson Innovative Medicine, Allegro Ophthalmics, Lineage Cell Therapeutics (CellCure Neurosciences), Roche, Cognition Therapeutics, Stealth BioTherapeutics, Annexon Biosciences, Astellas Pharma, Iveric Bio, Apellis Pharmaceuticals, among others.
Wet Age-related Macular Degeneration Market
Wet Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key wet AMD companies including EyePoint Pharmaceuticals, Inc., AbbVie, Caregen Co. Ltd., Exegenesis Bio, Shanghai Henlius Biotech, Skyline Therapeutics, 4D Molecular Therapeutics, Ocugenix Corporation, Adverum Biotechnologies, Inc., Ashvattha Therapeutics, Inc., AiViva BioPharma, Inc., Ocular Therapeutix, Inc., Clearside Biomedical, Inc., Hoffmann-La Roche, Kyowa Kirin, Inc., Opthea Limited, AffaMed Therapeutics Limited, EyeBiotech Ltd., Novartis, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
